medically compressed gas air system testing|medical gas field reporting requirements : traders Compressed medical gases (CMG or medical gases) include gaseous and liquid (cryogenic) forms stored in high-pressure cylinders that are administered as a gas. Types of compressed webWatch gene marquinez onlyfans on Erome porn videos xxx. Videos. . gene marquinez onlyfans. Subscribe (671) From: anonymous. 9 months ago 2,123 02:16. 95% (22 votes) .
{plog:ftitle_list}
WEBVHD was first demonstrated in 1978, and after numerous advertisements in 1981/1982 National Geographic magazines, it was eventually released in Japan on April 21, 1983. .
Testing your compressed air is simple with Trace Analytics. We’ll set you up with easy to follow instructions, comprehensive videos to guide you, a third-party distributer if you choose, and a .Compressed medical gases (CMG or medical gases) include gaseous and liquid (cryogenic) forms stored in high-pressure cylinders that are administered as a gas. Types of compressed
A good-size hospital with 200 beds and ten operating rooms can have a medical air system, a laboratory air system, and pneumatic air systems. The medical air systems must all follow the NFPA 99 guidelines.Trace Analytics, LLC is an ISO 17025 accredited laboratory specializing in the analysis of compressed air and gas. The laboratory can provide testing for health care facilities who maintain their own equipment with an experienced medical .
Analytical and risk-based testing methods are recommended for all pharmaceutical process gases and compressed air systems (ISPE, 2011). We monitor and detect target gases commonly found in compressed air systems of hospitals and medical facilities. In these systems, a medical air compressor produces air at 100 psig and feeds it at this same pressure to .
Trace provides medical air and gas analyses in support of NFPA 99 guidelines, directly to health care facilities who maintain their own equipment with an experienced medical gas and vacuum pipeline testing ASSE 6030 qualified .Bulk gas emergency (for orders, see Bulk Gas Support) Technical questions about cylinder labels; Have a gas leak? Evacuate IMMEDIATELY and call 911. Bulk Gas Support For Airgas National Carbonation CO 2 Orders: (800) 772-8144. [email protected].
%PDF-1.6 %âãÏÓ 17454 0 obj >stream hÞì›[¯$DZ ÿÊ~´ ¤‰[Þ€ :òƒ Ë„(? ‚0–F Š$x Ì ïìÚ½¾&gÆÃáåXÆQ¾ÄÄTDfu¯ŠªZ±;V-{²§Zþä6ö .
K6042 Breathing Air Gas Contaminant Testing Kit; K6099 Medical Gas Testing Kit; Manufacturing / ISO 8573 Air. K810 P:W:O Testing Kit; K811 Gases, P:W:O Testing Kit; . Proper assessment of the compressed air system, and regular testing can prevent serious contamination and promote the overall health of the system. Test your compressed air to .sufficient for the proper design of medical gas systems is hereby disclaimed. It is the intent of Amico Source Corporation that this Design Guide should only be used as a single tool . 5.2 Steps to Implementing the Medical Compressed Air System 52-66 5.2.1 Step 1: Discovery 52-53 5.2.2 Step 2: Design 53 5.2.3 Step 3: Plant Sizing 53-55The Dräger compressed air systems use 3 separate compressors, each with 2 redundant pressure tanks, filter systems, compressed air conditioners and pressure reducer stations. This makes maintenance and repairs possible without interruption of the air supply, offering additional operational security. In today’s highly industrialized society, countless processes require the use of compressed air. Industries including Oil & Gas, Petrochemical, Industrial, Environmental, Medical, Food & Beverage, Pharmaceutical, and Airline incorporate compressed air systems in their manufacturing cycles.
K6042 Breathing Air Gas Contaminant Testing Kit; K6099 Medical Gas Testing Kit; Manufacturing / ISO 8573 Air. K810 P:W:O Testing Kit; K811 Gases, P:W:O Testing Kit . and modifications of compressed air and gas systems should all be validated prior to use. Air or gas system validations become even more critical in the cases of pharmaceutical .

tensile test in universal testing machine
medical waste anesthetic gas system
TRI Air Testing proudly pioneered the science of compressed air and pure gas testing. TRI’s patented air/gas sampling equipment was designed in 1975 for the U.S. Navy Divers. Since then, TRI Air Testing continues to sponsor the U.S. Navy and Coast Guard D.C.A.T. program as well as other branches of the United States military. With purity, many parts of the pharmaceutical industry will use class 1 compressed gas based on the maximum number of permitted particulates. The particle limits are: Microbiological requirements . Although compressed gas and air systems are inhospitable environments, they can aid microbial survival if there are available nutrients.
A good-size hospital with 200 beds and ten operating rooms can have a medical air system, a laboratory air system, and pneumatic air systems. The medical air systems must all follow the NFPA 99 guidelines. We follow these guidelines, from the beginning, when we assess the demand for air in a hospital. [TRI Air provides equipment to test compressed air samples as part of air quality compliance, safety, SQF Medical Solutions for medical gas testing standards including NFPA 99 Analytical and risk-based testing methods are recommended for all pharmaceutical process gases and compressed air systems (ISPE, 2011). Put ISPE Good Practice Guide into Action The first and most critical step toward implementing the Good Practice Guide is to assess the risks associated with both your system and your product.
What is compressed air testing used for in industrial and O&G settings? It can have a massive impact on your uptime and bottom line. . This industrial air testing system is popular because it is both efficient and cost-effective to determine compressed air quality. . This method employs gas chromatography to detect compressed air .
Medical gas supply systems deliver CMGs to piped distribution systems at health care facilities. Oxygen USP (United States Pharmacopeia) and medical air USP provide direct assistance for breathing and also assist in the .e. medical compressed air for both respirable applications and surgical tools (at 400 kPa and 700 kPa respectively), f. oxygen/carbon dioxide mixture (5% CO 2), g. medical vacuum; h. waste anaesthetic gas scavenging systems (AGSS). 1.2 Throughout this volume, the phrase ‘Medical Gas Pipeline Systems’Medical Device Manufacturing facilities undergo FDA inspections to assure compliance with the Quality System Regulation (QS) requirements. [FDA-Quality System Regulation Guidance Document 21 CFR 820] Each manufacturer .
In most cases, end users select compressed air system components by comparing technical data from various air treatment manufactures. In 1991, the International Standards Organization (ISO) established the 8573 compressed air quality standard to facilitate compressed air system component selection, design and measurement.K6042 Breathing Air Gas Contaminant Testing Kit; K6099 Medical Gas Testing Kit; Manufacturing / ISO 8573 Air. K810 P:W:O Testing Kit; K811 Gases, P:W:O Testing Kit; . A medical device compressed air system should include a properly maintained compressor, adequate piping and distribution, and effective filters. It’s essential to properly .
Maria Sandoval, Microbiologist, Trace Analytics Trace Analytics is an A2LA accredited laboratory specializing in compressed air and gas testing for food and beverage manufacturing facilities. Using ISO 8573 sampling and analytical methods, their laboratory tests for particles (0.5-5 microns), water, oil aerosol, oil vapor, and microbial .
Microbial Testing of Compressed Air Micro Testing of Compressed Air or Bioburden Testing per ISO 8573-7 is generally conducted by the pharmaceutical, medical device and food industries. Microbial contaminants found in the compressor or compressed air lines can be devastating to a final product in these industries. A regular Micro Testing program can provide . Contact. Nex Flow Air Products Corp. 10520 Yonge Street, Unit 35B Richmond Hill ON., L4C 3C7, Canada. USA / Canada : 1 877-797-2777 International : 1 416- 410-1313 Open a Nex Flow Customer Support Ticket
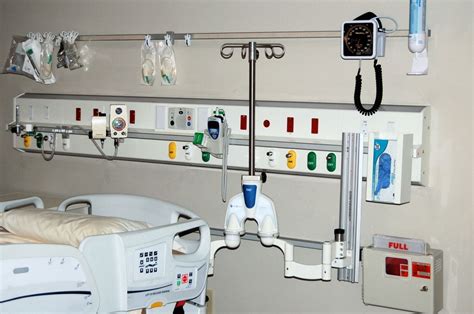
medical room gas connections
l Monitoring compressed air in manufacturing processes l Monitoring supplied breathing air l Monitoring medical compressed air systems l Meets OSHA Grade D breathing air and NFPA-99 requirements l Can custom design compressed air monitors for OEM applications www.enmet.com 1-800-521-2978 /1 | airbestpraties .o 3 SS MC S These include both Level 1 and Level 2 Medical Piped Gas and Vacuum Systems of NFPA 99. We monitor and detect target gases commonly found in compressed air systems of hospitals and medical facilities. In these systems, a medical air compressor produces air at 100 psig and feeds it at this same pressure to medical air dryers and filters.K6042 Breathing Air Gas Contaminant Testing Kit; K6099 Medical Gas Testing Kit; Manufacturing / ISO 8573 Air. K810 P:W:O Testing Kit; K811 Gases, P:W:O Testing Kit; . Regular testing of compressed air systems and other process gases that come into contact with pharmaceutical products is critical to ensuring the quality and integrity of the .
Test gases include: carbon dioxide (CO2), carbon monoxide (CO), compressed natural gas (CNG), helium (He), nitrogen (N2), and oxygen (O2) in pure and mixed form. Due to government regulations, shipping of compressed gases requires special handling and extra costs depending on the ship-to address. Please contact the Nuvair sales team for . OSHA Standard 29 CFR 1910.134(i)(1) These “breathing air” systems are designed to meet Occupational Safety and Health Administration (OSHA) Standard 29 CFR 1910.134(i)(1) which states: “Compressed breathing air shall meet at least the requirements for Grade D breathing air described in ANSI/Compressed Gas Association Commodity .
tensile test of mild steel on universal testing machine

WEBLeaksAndRumours (@LeaksAndRumours) is a Twitter account that shares the latest news and rumors about technology, gaming, entertainment and more. Follow them to stay .
medically compressed gas air system testing|medical gas field reporting requirements